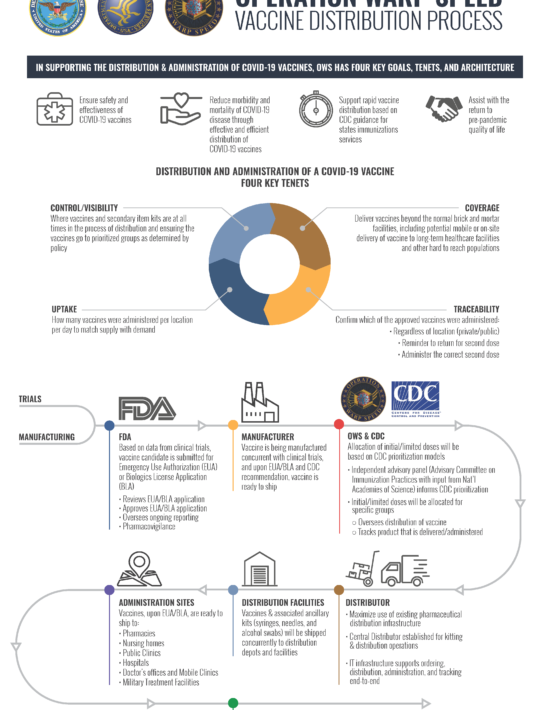
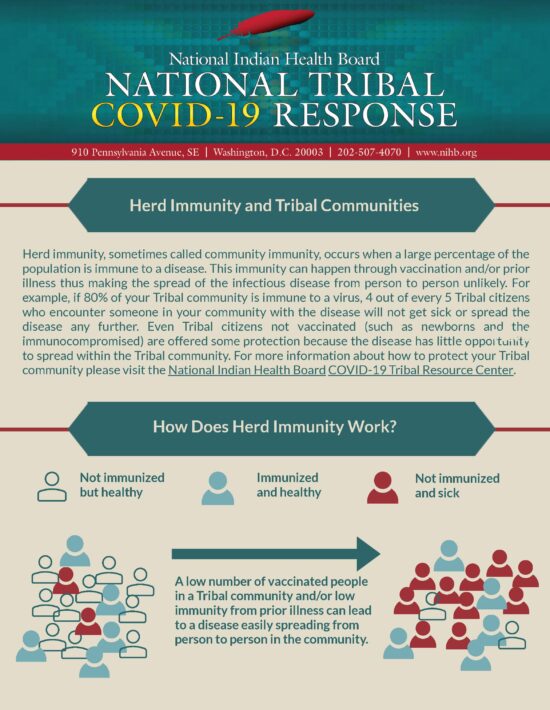
Vaccine Information and Tribal Support
The National Indian Health Board, in an effort to keep Tribes engaged and informed in the development of a COVID-19 vaccine, offers this platform that contains communications, resources and advocacy materials.
Home » Vaccine Information and Tribal Support
Advocacy Materials
Correspondence sent to Congress
Correspondence sent to the Administration and Federal Agencies
Federal Agency Resources and Communications
Centers for Disease Control and Prevention (CDC)
Centers for Disease Control and Prevention (CDC) V-safe After Vaccination Health Checker – Healthcare systems and providers have a vital role in encouraging COVID-19 vaccine recipients to participate; please see recommended provider/patient script below and attached v-safe information sheet and poster. In addition, CDC’s v-safe web pages provide information on how to register and complete a v-safe health check-in (including step-by-instructions with images), troubleshooting, FAQs, and contact information for technical support. These web pages will be continuously updated with additional resources.
Suggested provider messaging “CDC has created a way for you to report how you feel after COVID-19 vaccination through a smartphone-based tool that uses text messaging and web surveys to check in with you. Here (or in your packet) is a v-safe information sheet with more details and simple instructions to sign up.”
Centers for Medicare and Medicaid Services (CMS)
Vaccine Toolkit
A new COVID-19 vaccine coverage and reimbursement toolkit was released by CMS on March 15, 2021. The updated toolkit provides the rate increase in Medicare vaccine administration, expansion of vaccine eligible individuals and administration coverage, as well as 100 percent FMAP for COVID-19 vaccines and administration. CMS also provides Medicaid, Children’s Health Insurance Program (CHIP), and Basic Health Plan (BHP) tools for states to consider coverage and reimbursement for the vaccine administration. CMS addresses issues in the toolkit, such as:
- Clinical and operational considerations for COVID-19 vaccines and vaccination planning under Medicare, Medicaid, and CHIP;
- Medicaid coverage of COVID-19 vaccines and vaccine administration under the Families First Coronavirus Response Act (FFCRA);
- Medicaid vaccine administration coverage, reimbursement, and cost sharing policies for adults (including limited beneficiaries) and for children;
- Coverage reimbursement under CHIP and BHP;
- Reporting requirements;
- Provider enrollment; and
- Education and outreach.
NIHB Releases Video Explainer on the Vaccine Mandate for Staff of Medicare and Medicaid-certified Facilities
On November 5, 2021, the Centers for Medicare and Medicaid Services (CMS) published a regulation regarding vaccination requirements for staff of Medicare and Medicaid-certified facilities. It aims to protect those fighting this virus on the front lines while also assuring individuals and their families that they will be protected when seeking care.
This emergency regulation is effective as of November 5, 2021. It provides guidance on staff vaccination requirements applicable to Medicare and Medicaid-certified facilities that are regulated under the Medicare Conditions of Participation (CoPs), which includes Indian Health Service (IHS) facilities. However, enforcement of this regulation has been halted in response to injunctions issued as a result of the court cases discussed in this video.
US Food and Drug Administration (FDA)
US Department of Health and Human Services (HHS)
Health Resources and Services Administration (HRSA)
Webinar Recording, Addressing Vaccine Hesitancy 2/3/21: Link to Recording
Passcode: n1c=*y70
Indian Health Service (IHS)
National Institutes of Health (NIH)
White House
Webinar Recordings and Presentations
NIHB Webinar: COVID-19 Vaccine Planning Information Session for Tribal Communities 9/25/20
NIHB All Tribes Call: Vaccine Plan Informational Webinar 10/30/20
NIHB hosted an informational call on Friday, October 30 to learn more about the Indian Health Service’s (IHS) COVID-19 Pandemic Vaccine Draft Plan which was released October 14. Agency representatives provided an overview of the plan, shared a summary of Tribal comments they have received on it, and outlined an expected timeline for finalizing the plan. Representatives from the Centers for Disease Control and Prevention (CDC) also joined the call to provide information on the process states are following to submit their vaccine plans, and an update on the progress to date.
Understanding Diverse Communities to Support Equitable and Informed COVID-19 Vaccination Decision-Making
Presented by the National Association of County and City Health Officials (NACCHO), National Indian Health Board (NIHB), The Association of State and Territorial Health Officials (ASTHO), The Association of Immunization Managers, and the Institute for Vaccine Safety at the Johns Hopkins Bloomberg School of Public Health.
Tribal Self Governance Advisory Committee (TSGAC) Webinar on Tribal Best Practices in Vaccine Distribution
Vaccination in Tribal Communities
Resources for Tribal Communities from the National Indian Health Board
Other Resources
Understanding Diverse Communities and Supporting Equitable and Informed COVID-19 Vaccination Decision-Making Report – Wave 3 Findings
The report from Wave 3 of Understanding Diverse Communities and Supporting Equitable and Informed COVID-19 Vaccination Decision-Making, is the result of a joint effort by national organizations, including the National Indian Health Board, aimed to support health departments and Tribal governments with information and perspectives from communities about their current COVID-19 vaccination decisions.
The project was designed in three waves, occurring between December 2020 and July 2021. Findings from Wave 1 (conducted November 1 – December 21, 2020) featured results from a 2,525-person panel survey and 25 online community conversations and real-time polling with ~400 adults, including local Native American, African American, and Latinx communities. In Wave 2, the same individuals were engaged in a second set of community conversations (January 26 – February 13) to understand what has changed for them, and whether or how these changes influenced their decision-making. Wave 3 (May 1-22, 2021), continued the conversation with the same individuals from the previous waves.
The report features a snapshot of both qualitative perspectives and real-time polling from the participants, along with considerations for how health departments and Tribal governments can use these findings, collaboratively with their community partners, in their efforts to support equitable COVID-19 vaccine uptake. Read more here.
Expert Opinion Articles
The articles below were written by medical or health policy experts. While they have been vetted to ensure that the author is an appropriate subject matter expert, the opinions expressed do not necessarily reflect the opinions of the National Indian Health Board, our board, or our partners. These articles are being provided for educational purposes only.

Contact Us
For more information or questions, please contact Chris Chavis, Policy Center Director, at [email protected].
For media inquiries, please contact Janee Andrews at [email protected].
© Copyright 2020, National Indian Health Board. All Rights Reserved. Website by DrawBridge Creative.
This website is supported by the Centers for Disease Control and Prevention of the U.S. Department of Health and Human Services (HHS) as part of financial assistance awards totaling $2,000,000, Tribal Capacity Building for COVID-19 Disease Control, and $4,000,000, Addressing COVID-19 Vaccine Confidence Through Tribal Health Departments, with 80 percent funded by CDC/HHS, and 20 percent funded by non-government source(s). The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by CDC/HHS, or the U.S. Government.